
Navigating supplement facts label requirements can be complex, but compliance is non-negotiable for manufacturers. A supplement facts label provides detailed information about the product’s ingredients, nutrients, and serving size, ensuring transparency and consistency. These labels aren’t just a legal requirement—they’re a vital tool for helping consumers make informed decisions about the products they trust.
The Food and Drug Administration (FDA) plays a key role in setting labeling standards to protect public health and ensure accurate product representation. In this guide, we’ll break down everything you need to know about FDA supplement facts labeling requirements, including mandatory labeling elements, formatting guidelines, common compliance mistakes, and special considerations for manufacturers. We’ll also explore how tools like Food Label Maker can simplify the process, helping you create nutrition labels that are accurate and compliant with ease. You can explore our pricing plans here.
See How FoodLabelMaker Can Help You
Core Differences Between Supplement Labels and Nutrition Labels
At first glance, supplement facts labels and nutrition facts labels may look similar, but these two types of information panels serve very different purposes. A nutrition facts label is designed for conventional foods and beverages and focuses on the product’s calorie count and nutrient content, like fat, sugar, and protein. It also lists any allergens present in the food and makes relevant health claims if necessary.
In contrast, a supplement facts label is required for dietary supplements like vitamins, minerals, and botanical products. It details the ingredients and their amounts to help consumers understand what they’re putting into their bodies. While the FDA regulates both, their target products and legal requirements differ significantly.
For example, a protein powder marketed as a dietary supplement needs a supplement facts label listing its key ingredients, like whey protein isolate and added vitamins. However, a ready-to-drink protein shake, classified as a conventional food, needs a nutrition facts panel because it is consumed as a food product rather than a dietary supplement. Understanding these FDA label differences is essential for ensuring compliance and avoiding potential mislabelling issues.
When to Use Supplement Facts Labels

Not every product falls under the category of dietary supplements, and understanding the distinction is critical for compliance. According to the FDA, dietary supplements are products, ingested orally, that contain one or more dietary ingredients, such as vitamins, minerals, herbs, amino acids, or other substances intended to supplement the diet.
To determine if your product qualifies as a dietary supplement, consider its ingredients and intended use. Supplements are meant to add nutritional value or provide specific health benefits, distinguishing them from conventional foods and beverages.
Examples of products requiring supplement facts labels include:
- Vitamin C tablets or multivitamins
- Herbal teas marketed as supplements
- Fish oil capsules or omega-3 supplements
- Protein powders labeled as dietary supplements
- Botanical extracts, such as echinacea or ginkgo biloba
Each of these products must meet FDA supplement criteria to ensure proper dietary supplement labeling, protecting both manufacturers and consumers.
FDA-Required Elements for Supplement Facts Labels
Creating a compliant supplement facts label isn’t just about following best practices—it’s a legal requirement set by the FDA. These labels provide critical information that helps consumers make informed decisions and ensures transparency in your product’s content.
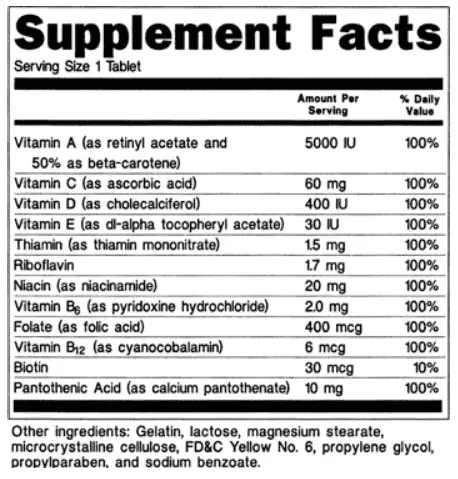
Mandatory Information: What the FDA Requires
A compliant supplement facts label must include specific elements mandated by the FDA:
- Title: The label must begin with the title “Supplement Facts” to identify the product as a dietary supplement.
- Serving Size and Servings Per Container: Clearly state the recommended serving size and the total number of servings in the package.
- Nutrient Content: Include all nutrients, their quantities, and the percent Daily Value (%DV) based on a 2,000-calorie diet.
- Dietary Ingredients List: List all ingredients in descending order of weight, ensuring transparency for consumers.
- Disclaimers: For structure/function claims (e.g., “supports immune health”), add the required disclaimer: “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
Layout and Design Rules
The FDA dietary supplement labeling guide details the strict requirements for the format and presentation of dietary supplement products’ labels to ensure they are clear and readable:
- Title and Hairlines: The title “Supplement Facts” must be in bold, larger than any other text in the panel, and span the full width whenever practical. The panel must be enclosed in a box using hairlines to separate each dietary ingredient.
- Font and Style: Use a single, easy-to-read type style. The text must be black or in one color, printed on a white or neutral contrasting background. Upper- and lowercase letters should be used, except for small packages, where all uppercase letters are allowed.
- Type Size: Except for small packages, the general text must be no smaller than 8 points, while headings and footnotes can be 6 points. The title “Supplement Facts” must be in a larger type size than all other text.
- Spacing and Legibility: Ensure at least one point leading (spacing between lines) and that letters do not touch each other. Hairlines must separate ingredients for clarity.
- Aggregate Labels: For products with multiple packets (e.g., morning and evening doses), the label can list each packet’s information in separate columns or use an aggregate format to display all details cohesively.
Common Misconceptions About Label Elements
Even with clear FDA guidelines, manufacturers often misunderstand key requirements for supplement facts labels, leading to costly compliance issues. Here are two common misconceptions:
- Optional vs Mandatory Elements: A frequent mistake is assuming certain details, like serving size or % Daily Values, are optional. These elements are mandatory for most products, and omitting them can result in non-compliance. It’s crucial to distinguish what must be included versus what is optional, such as the inclusion of additional nutrients or nutrient content claims such as “high in vitamin C”.
- Displaying Proprietary Blends: While proprietary blends are allowed, many manufacturers fail to list the total weight of the blend or display ingredients in descending order by weight. Additionally, failing to clearly indicate that individual ingredient quantities within the blend are not disclosed can create compliance issues. Properly labeling blends avoids confusion, particularly for nutrients like dietary fiber or vitamin C.
Common Compliance Mistakes to Avoid
Even with the best intentions, compliance mistakes on supplement facts labels are common and can lead to serious consequences, including fines, product recalls, or damage to your brand’s reputation. Below are some of the most frequent errors and how to avoid them:
Misleading Claims and Misrepresentation
One of the biggest pitfalls for manufacturers is making unsupported health claims. For example, statements like “cures illness” or “prevents disease” are prohibited unless specifically approved by the FDA. Even structure/function claims, like “supports immune health,” must include the required disclaimer. Misrepresentation not only misleads consumers but also exposes your business to regulatory action, including costly fines and product recalls.
Formatting Errors
Formatting mistakes often go beyond simply not having the correct font sizes and contrast—they can affect the usability and accuracy of your label. For example, forgetting to include required hairlines to separate dietary ingredients or failing to align text correctly within the supplement facts panel can make the label difficult to read and cause non-compliance.
Errors in alignment or spacing can lead to critical information being overlooked, confusing consumers, and increasing the risk of regulatory scrutiny. Additionally, using inconsistent formats across different products in your range may erode consumer trust and make it harder to meet FDA standards for clarity and presentation.
Failure to Update Labels
Regulations and product formulations change over time. It’s important to update your labels accordingly. For example, introducing a new ingredient, adjusting serving sizes, or modifying nutrient content without revising the supplement facts panel can lead to inaccuracies that put your product at risk of non-compliance.
Proactive label updates ensure compliance and provide consumers with accurate and up-to-date information. Regularly reviewing your labels—especially when introducing new products or making changes to existing ones—is an essential step in maintaining a compliant and successful brand.
Special Requirements for Supplement Manufacturers

Supplement manufacturers face unique labeling challenges that go beyond standard FDA requirements. From proprietary blends to ensuring consumer safety, these special considerations are designed to promote transparency and accuracy.
Proprietary Blends Labeling Rules
Proprietary blends often include multiple ingredients combined into a single formula. While manufacturers are not required to disclose the exact amount of each ingredient, the FDA mandates listing the total weight of the blend and presenting the ingredients in descending order by weight. These ingredients should be accompanied by a symbol that directs to the footnote stating, “Daily Value Not Established.”
Adverse Event Reporting and Consumer Safety
Labels play an important role in consumer safety, especially when it comes to addressing adverse events or reactions. Manufacturers are legally required to report any serious adverse events associated with their products, and proper labeling supports this process by providing clear instructions for use and contact information for reporting issues. Transparent labeling not only meets legal obligations but also reinforces supplement safety.
GMP Compliance and Its Impact on Labelling
Good Manufacturing Practices (GMP) set the foundation for product quality and accuracy in labeling. GMP compliance ensures that labels reflect the actual contents of the product, preventing discrepancies that could lead to non-compliance. For example, GMP guidelines require accurate measurements of dietary ingredients and proper documentation of production processes to ensure that the supplement facts panel is reliable.
Staying compliant with GMP also demonstrates a commitment to product quality and safety, fostering trust among consumers and regulators alike.
How Food Label Maker’s Tools Simplify Compliance
Managing dietary supplement facts labeling can be complex due to stringent FDA regulations and evolving industry standards. Food Label Maker offers specialized supplement and nutrition label maker tools to streamline this process for manufacturers. Our supplement labeling tools include:
- FDA-Compliant Supplement Facts Labels: Quickly create accurate labels that meet all FDA requirements for dietary supplements.
- Custom Ingredient Setup: Add proprietary or unique ingredients to your database, ensuring accurate and transparent labeling.
- Easy Formula Builder: Streamline the process of creating supplement formulations with a user-friendly interface.
- Overage & Moisture Loss Management: Accurately account for overages and moisture loss to ensure compliance and formula consistency.
- Editable Ingredient & Statement List: Customise your ingredient lists and required statements while maintaining compliance with FDA regulations.
- Automated Allergen Detection: Automatically scan your formula to identify and highlight allergens for precise labeling.
- Notes & Attachments: Attach critical documents and add notes to formulas for streamlined communication and record-keeping
- Formula Costing: Easily calculate the cost of your supplement formulations for better financial planning.
- Customizable Label Format: Format your labels to meet your specific needs by choosing what information to include or omit, all while staying compliant with FDA guidelines.
By leveraging these features, supplement manufacturers can efficiently navigate the complexities of FDA compliance, reduce the risk of errors, and focus on delivering high-quality products to consumers.
Conclusion
Creating accurate, FDA-compliant supplement facts labels is essential for protecting consumer safety and ensuring your product meets regulatory standards. Proper labeling not only demonstrates your commitment to transparency but also safeguards your business from potential fines or recalls.
By using professional labeling tools like Food Label Maker, manufacturers can streamline the process, reduce errors, and produce labels that meet every requirement.
Whether you’re crafting a supplement facts label or managing a complex product portfolio, these tools offer the efficiency and precision needed to succeed in the competitive supplement industry. View our pricing plans and start creating effective food labels today.
FAQs :
Can I list proprietary blend ingredients without disclosing quantities?
Yes, the FDA allows manufacturers to list proprietary blend ingredients without disclosing their exact quantities. However, the total weight of the blend must be included, and the ingredients must be listed in descending order by weight within the blend.
While individual amounts can remain undisclosed, transparency is still required to help consumers make informed choices.
What happens if my labels don’t comply with FDA requirements?
Non-compliance with FDA labeling requirements can lead to serious consequences, including product recalls, warning letters, fines, or legal action. Mislabelled products may also face delays at customs or removal from store shelves, damaging your brand’s reputation and consumer trust.To avoid these issues, ensure your labels meet all FDA guidelines, including accurate ingredient listings, serving sizes, and required disclaimers. Using professional tools like Food Label Maker can help you create compliant labels and minimize the risk of errors that could impact your business. Visit their regulatory hub to find out more about FDA requirements.



