Did you hear about the latest FDA policy change that’s shaking up food imports?
On July 9, 2025, the FDA released updated guidelines on the De Minimis Exemption. The long-standing rule will be suspended, meaning all FDA-regulated products, including medicines, food, beverages, dietary supplements, cosmetics, medical devices and biological samples, regardless of value, will face full FDA review at the border. This makes accurate and compliant nutrition labeling more critical than ever.
The administration of President Trump has made broader trade policy a focus, which includes the elimination of the de minimis shipments exemption for many imported goods. Not sure what this significant shift means for your products? You’re not alone. This article will break down the new requirements, particularly for food imports and nutrition facts labeling, and show you exactly how to avoid costly detentions and delays.
Want a sneak peek first?
What is the De Minimis Exemption?
Here’s how the National Foreign Trade Council (NFTC) defines the de minimis rule:
“The De Minimis Tax Exemption is a law that allows shipments intended for American businesses and consumers, valued under $800 per person per day, to enter the U.S. free of duty and taxes.”
This means that food imports less than $800, which were once considered duty-free, could essentially “slip-through” US Borders without FDA inspection, potentially allowing non-compliant food labels to enter the market.
Why Did the De Minimis Exemption End?
As of 9 July 2025, all international postal shipments and other imported goods will be actively examined by customs and border protection officials. With the removal of this exemption, every shipment will now be subject to applicable tariffs and a full FDA review. Compliance is now knocking on your door, and it’s loud!
Here’s a brief overview of the FDA Review and Inspection Process:
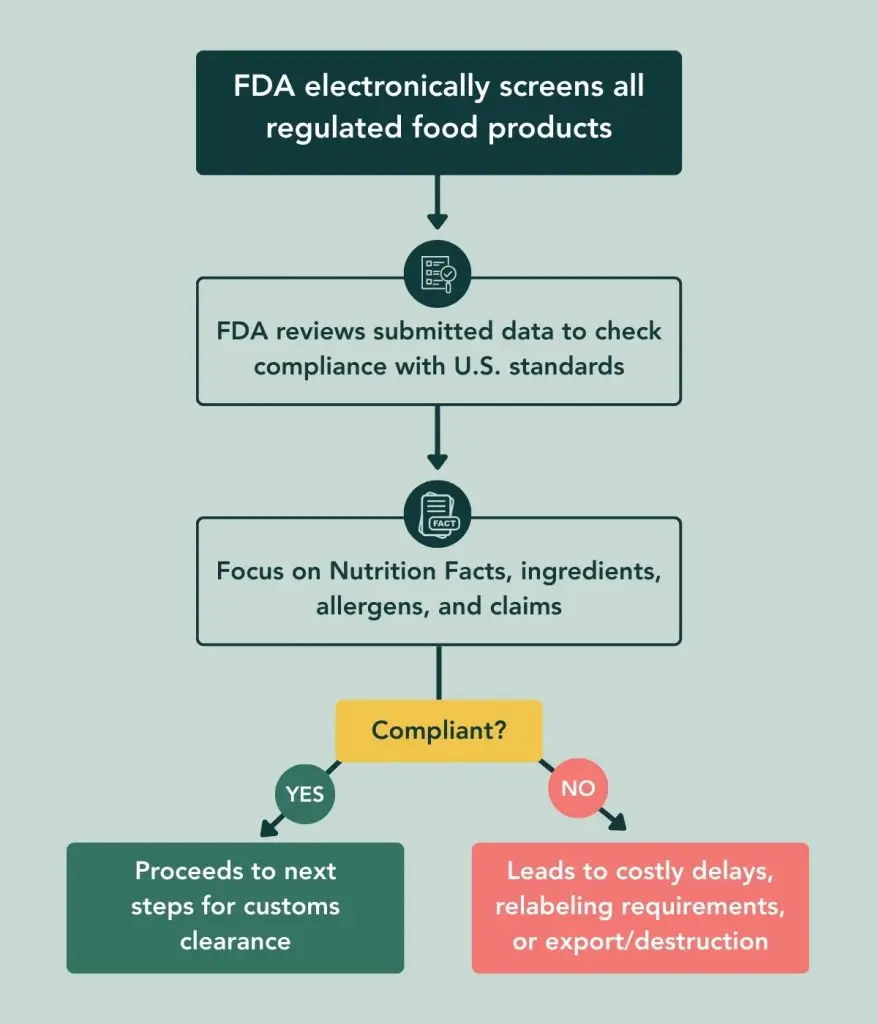
If you’d like more of a deep dive into the requirements for FDA import labeling, read our guide on FDA Import Labeling: Compliance Guide for F&B Newcomers.
See How FoodLabelMaker Can Help You
Nutrition Labeling: Now More Critical Than Ever
Gone are the days when a less-than-perfect nutrition label might slip through. With the end of De Minimis, the FDA’s new framework demands that importers have adequate systems in place to ensure compliance from the start. With this new FDA-import framework, the regulatory accuracy and depth of your nutrition information is exactly what will be reviewed and inspected during customs clearance.
Under the Microscope: FDA’s Primary Labeling Targets
Here are the key areas the FDA will be rigorously checking:
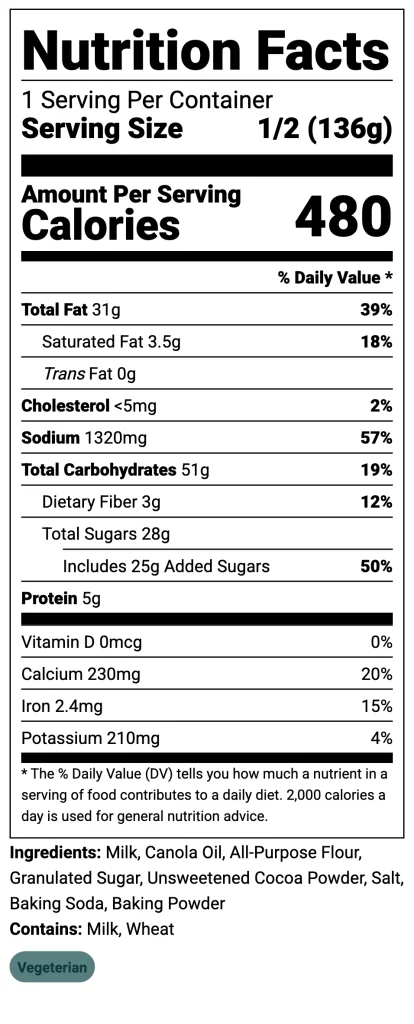
- Nutrition Facts Panel: Ensure it’s accurate and uses the updated format, including mandatory “Added Sugars,” Vitamin D, and Potassium.
- Correct Ingredient Declarations: Ingredient lists must be complete and in precise descending order by weight.
- Allergen Labeling: All major food allergens (including sesame) must be clearly and correctly declared for consumer safety.
- Truthful & Non-Misleading Claims: Any claims like “healthy” or “gluten-free” must be scientifically substantiated and not misleading.
The Cost of Ignoring FDA Regulations
Considering all these changes, you’re probably wondering what would happen if your label doesn’t fully comply. Unfortunately, the new FDA import rules leave very little room for error.
The FDA has given no grace period for non-compliance with the updated program, meaning immediate enforcement actions are a real possibility for labels that aren’t compliant. Outdated labeling practices are now a major gamble, with proactive compliance being your best way forward.
Non-compliant labels now face a dramatically higher chance of immediate detection and import detentions at the border, leading to:
- Significant added costs from storage and re-labeling
- Applicable tariffs on goods while held on the border
- Major delays in getting your products to market
- Potential damage to your brand’s reputation
In short, ignoring these new requirements can severely impact your business on a global basis.
Achieve Labeling Peace of Mind with Food Label Maker
Navigating this new FDA-Regulated Import reality can feel like a maze. The good news is you don’t have to do it alone.
Our Expertise
Food Label Maker are experts when it comes to FDA Regulations, as our software is always up-to-date on everything, so you don’t have to be. Our dedicated regulatory team stays ahead of the curve by proactively monitoring and implementing changes as soon as they’re announced. This allows us to translate complex legal jargon into clear, actionable labeling solutions for your products.
Tools & Services
Our easy-to-use platform takes the guesswork out of food labeling compliance, with accurate nutrition analysis, and a smart algorithm that automatically identifies allergens in your recipe for proper declaration and suggests possible nutrition claims that align with your product.
You’ll also have access to over 20 customizable label formats with the flexibility to choose which nutrients to display, giving you control over your label’s final look while always maintaining compliance for your specific market needs.
On top of that, you can create, scale, and duplicate recipes with a single click, providing a detailed nutrition breakdown of all your ingredients and generating a comprehensive recipe card for your team.
From Challenge to Confidence
Ultimately, the most valuable tool Food Label Maker offers is peace of mind. By providing the expertise and features needed for perfect, compliant labels, we help you avoid costly detentions and ensure a smooth, worry-free entry for your products into the U.S. market. This allows you to focus on growing your business, not on navigating complex regulations.
Final Thoughts on the De Minimis Exemption End and Food Import Compliance
The new FDA import reality is clear: the de minimis exemption is gone, and all food shipments now face rigorous scrutiny at the border. So, your nutrition facts, ingredient declarations, allergen labeling, and product claims are now under the spotlight. With the risk of costly detentions now higher than ever, proactive compliance is the only way to ensure a smooth import process. The removal of this exemption, which has been a focus of President Trump, isn’t just a concern for products from specific regions; it impacts items from all other countries, too.
To help you navigate this new reality, you can hire one of our nutrition experts and have your labels crafted, with compliance, from start to finish.
Our proactive approach means we don’t just react to changes; we anticipate them. We’ve seen countless labeling scenarios and know how to predict potential issues, ensuring your labels are future-proofed against common pitfalls.
Frequently Asked Questions About the End of the De Minimis Exemption
When does the De Minimis Exemption end in the U.S?
The De Minimis Exemption was suspended on July 9, 2025. It came after the FDA released a statement announcing that all FDA-regulated products must be submitted to the FDA for review. The end of the exemption means that all imported goods will be actively examined by border protection officials.
What types of goods are most commonly imported under the De Minimis Exemption?
Under the de minimis exemption, FDA-regulated imports under the value of $800 were not subject to a full FDA border review. These goods included medicines, food, beverages, dietary supplements, cosmetics, medical devices and biological samples.
What are the main reasons behind the proposed changes to the De Minimis Exemption?
The main reasons behind rescinding the De Minimis Exemption are to ensure that the health and safety of consumers is the top priority when it comes to imported goods. This applies to nutrition labeling as the De Minimis Exemption would allow non-compliant nutrition labels to potentially pass through the borders without strict FDA scrutiny. However, now that the rule has been suspended, all FDA-regulated products will be under review by FDA agents regardless of their monetary value.


