The importance of accurate and compliant labeling in the realm of food products cannot be overstated. The Food and Drug Administration (FDA) has established stringent laws and regulations that govern the packaging and what information is required on food labels. These laws are designed to ensure that consumers have access to vital information about the products they consume, and further promote transparency and safety when it comes to marketing a food product.
In this article, we will delve into the intricacies of these regulations, providing you with a comprehensive understanding of the fundamentals of food product labeling and packaging. Whether you’re a seasoned food manufacturer or a budding entrepreneur in the food industry, this guide will equip you with the knowledge you need to create ingredient labels that meet FDA standards and serve your customers’ needs.


Types of Panels
In the realm of food product labeling, there are three main types of panels that are crucial to understand:
- The Principal Display Panel
- The Informational Panel
Each of these panels serves a unique purpose and has specific requirements for what information must be displayed and how it should be formatted.
Principal Display Panel

The Principal Display Panel (PDP) is the front of the packaging that is most likely to be displayed or examined at retail. This panel is of utmost importance as it is the first point of contact between the consumer and the product.
- Placement:
The common name of the food, also known as the Statement of Identity, must be placed prominently on the PDP. This could be the legal name of the food, the common name, or a description of the food if the other two are not appropriate.
The Net Quantity of Contents, which is the amount of food in the package, is placed in the bottom 30 % of the PDP.
Information Panel

The Information Panel contains the Nutrition Facts Label, Ingredient Statement, Allergen Declaration, and the name and address of the manufacturer, packer, or distributor. This panel provides detailed information about the product that is essential for consumers to make informed choices.
- Placement:
The Information Panel is displayed immediately to the right of the Primary Display Panel (PDP), or the next available panel to the right if the adjacent one is unusable due to package design.
The Nutrition Facts Label and the Ingredient Statement are also found on the Information Panel. They must be placed on the same panel as the manufacturer’s information.
The Ingredient Statement should start after the word, “Ingredients” and the ingredients must be listed in descending order by weight.
The Allergen Declaration, which lists any major food allergens, can be placed anywhere on the Information Panel.
Statement of Identity

The Statement of Identity, also known as the common name of the food, is a crucial element of food labeling. It refers to the established name of the food product, if it has one.
If the food product doesn’t have a common name, a descriptive name that accurately represents the food product can be used. Misrepresenting a food product with a new name when it already has an established name could lead to confusion and is considered misleading.
If the food is available in various forms like “sliced” or “unsliced”, “whole” or “halves”, the label must specify the form contained in the package.
Please note that brand names are not considered or labeled as statements of identity, so the brand name should not be larger than or overshadow the size of the statement of identity.
- Placement
The Statement of Identity must be prominently displayed on the Principal Display Panel (PDP), parallel to the package’s base, and at the front of the packaging as this is most likely to be displayed or examined at retail.
The artwork on the packaging must also not obstruct the visibility of mandatory label information, or inaccurately represent the food. This ensures that the consumer can easily identify the food product at the point of purchase.
Net Quantity of Contents

The Net Quantity of Contents refers to the total amount of the food product contained within the packaging. This should be expressed in terms of weight, measure, numerical count, or a combination of these, depending on the nature of the food product. This information is crucial as it provides consumers with a clear understanding of the quantity of the food product they are purchasing.
Calculate the net weight by taking the average filled weight of the container and subtracting the average weight of the empty container, along with its lid and any additional packing materials. If the product is in liquid form, it must be described as gallons, quarts, pints, and fluid oz.
- Placement
The Net Quantity of Contents must be displayed within the lower 30% of the Principal Display Panel (PDP), and the amounts should be written after the words “Net Weight”.
The information should also be printed horizontally, parallel to the base of the packaging. This placement ensures that the information is easily visible to consumers at the point of purchase.
Nutrition Facts Label

The Nutrition Facts Label is a crucial component of food packaging that provides detailed information about the nutritional content of the food product. This includes information about the number of calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, and potassium contained in the food product.
This information is essential for consumers to make informed dietary choices. For a more comprehensive understanding of how to create an FDA-compliant food label, you can refer to our in-depth article on How to Get an FDA-Compliant Nutrition Facts Label for Food Products.
- Placement
The Nutrition Facts Label should be placed on the Principal Display Panel (PDP) or the Information Panel of the food packaging.
For packages where both the PDP and information panel lack sufficient space, the Nutrition Facts label can be positioned on any other visible panel. It should be easily visible and legible to consumers.
The placement of the Nutrition Facts Label is governed by FDA regulations and it’s important to ensure that your food packaging complies with these regulations.
Ingredient List


The Ingredient List is a crucial part of food labeling. It provides consumers with information about what’s inside the food product they’re purchasing.
The FDA requires that ingredients be listed in descending order by weight. This means the ingredient that weighs the most is listed first, and the ingredient that weighs the least is listed last. If an ingredient is made up of sub-ingredients, these can be listed in parentheses after the main ingredient.
For a more detailed explanation on how to create an FDA-compliant food label, you can refer to this article.
- Placement
The Ingredient List can be located on the same panel as the name and address of the manufacturer, packer, or distributor, either on the Principal Display Panel (PDP) or the information panel.
Its position can be before or after the Nutrition Facts label. It should start with the word “Ingredients” followed by a list of all the ingredients used in the product.
Allergen Statement

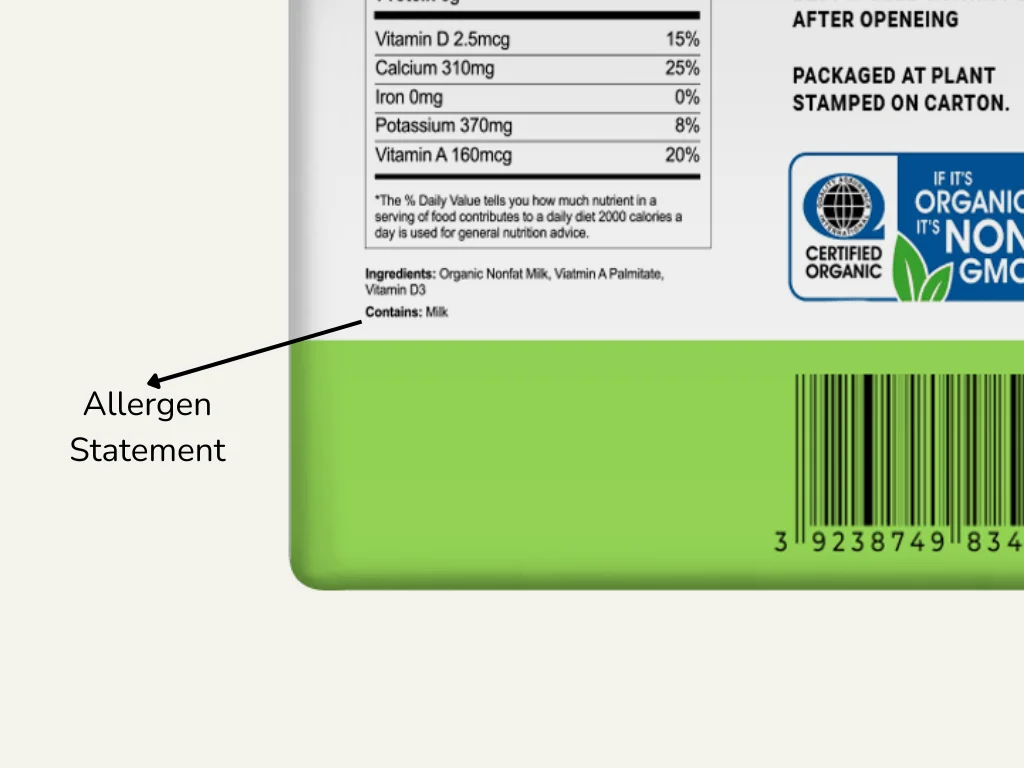
The Allergen Statement is another vital part of food labeling as it is designed to protect consumers who have food allergies. The Food Allergen Labeling and Consumer Protection Act requires that any major food allergens be declared on the food label so that consumers are aware of what they are consuming and whether an ingredient will cause them to have an allergic reaction. This includes allergens to items like peanuts, milk, and sesame. For a more in-depth look at allergen declaration, you can refer to this article.
- Placement
The Allergen Statement can be placed in various ways. It can be included in the ingredient list, for example, “peanut butter (peanuts)” or it can be stated separately, for example, “Contains peanuts, milk, and sesame.”.
Manufacturer, Packer, Or Distributer

The name and location of the manufacturer, packer, or distributor must be included on the food label. If the product is not manufactured or distributed by the person or company whose name appears on the label, then the relationship to the product should be stated, e.g., “manufactured for” or “distributed by”.
- Placement
The name and location of the manufacturer, packer, or distributor should be placed on the Information Panel. The location requirement must be the street address, city, state, and zip code. However, if the address is available in the telephone book, then you are allowed to list only the ZIP code.
What Else Can You Put On Your Packaging?
Beyond the mandatory elements of food product labeling, there are additional features you can include on your food packaging to provide more information to consumers and enhance the appeal of your product. These elements, while not required, can help your product stand out on the shelves and provide additional value to your customer base.
Nutrient Content Claims

Nutrient content claims are voluntary statements that highlight the presence or absence of certain nutrients in your product. These claims can be a powerful tool to attract health-conscious consumers. For instance, you can highlight that your product is “low in sodium” or “high in fiber”.
However, it’s important to note that these claims must be valid and comply with specific FDA labeling requirements for food. They can be displayed on the Principal Display Panel, Information Panel, or anywhere else on the package as long as they don’t interfere with the mandatory labeling elements.
- Placement
While there’s flexibility in where you can place nutrient content claims, it’s crucial to ensure they are easily visible and readable. A common practice is to place them on the front of the package (Principal Display Panel) where they can be easily seen by consumers.
Barcode

Barcodes are an essential element of food packaging, even though they are not a requirement by the FDA. They facilitate inventory management and point-of-sale transactions. A barcode contains a product’s unique identifier, known as the Universal Product Code (UPC).
- Placement
The barcode is typically placed on the back or side of the package where it doesn’t interfere with the design or mandatory labeling elements. It should be easily scannable, so it’s important to ensure it’s printed clearly and not distorted in any way.
Best Before Date

While not a federal requirement, some states require a “best before”, “expiration”, or “sell-by” date on certain food products. This date helps consumers know how long a product is expected to retain its optimal quality.
- Placement
The date should be placed where it doesn’t interfere with mandatory labeling elements. It must show the month, day, and year immediately adjacent to an explanatory phrase such as “best before,” “sell by,” etc.
Remember, while these additional elements can provide more information and enhance the appeal of your product, they should never compromise the clarity and compliance of the mandatory labeling elements. Always ensure your food labels adhere to FDA regulations to avoid any potential legal issues.