Health data from nutrition facts labels is often dismissed as niche information for those with specific dietary restrictions. But
In early 2025, the FDA introduced its proposed FOP nutrition label, often called the “Nutrition Info box.” This system moves critical data from the back of the box to the front. As consumer demand for healthier food choices rises, transparent FOP labeling is shifting from a regulatory hurdle into a vital opportunity to build brand trust.
This guide explores the current landscape of voluntary FOP labeling and breaks down the FDA’s new proposed standards. We’ll also share best practices for implementation, showing how transparency can sharpen your competitive edge.
Ready to get ahead of these regulations? Create a free label today or check out our pricing plans.
TLDR:
- Background: The FDA is shifting from voluntary to mandatory front-of-pack labeling to help consumers make healthier food decisions at a glance.
- The Goal: To combat diet-related chronic disease by moving “nutrients to limit” from the side Information Panel to the Principal Display Panel (PDP).
- The Proposed Standard: A standardized “Nutrition Info” box highlighting Saturated Fat, Sodium, and Added Sugars using “Low/Med/High” interpretive markers.
- The Timeline: As of early 2026, the proposed FOP nutrition label (the “Nutrition Info Box”) has not been finalized yet, but it is a key priority for federal health regulators.
- Key Exemptions: Small packages (under 12 sq. inches) and dietary supplements are currently proposed as exempt from mandatory FOP requirements.
- Brand Advantage: 79% of consumers check front-of-pack data. Adopting these dietary guidelines early reduces future compliance risks and builds immediate consumer trust.
- Next Step: Ensure your underlying nutrition facts label data is 100% accurate today to make the future transition to FOP labeling seamless.
Understanding FDA Front-of-Pack (FOP) Labeling
For decades, the Food and Drug Administration (FDA) has relied on the Nutrition Facts label as the primary tool for nutrition labeling.
While these side-panel labels provide essential data on calories and %DV, they often lack the quick, front-facing information needed for fast-paced shopping. To date, any front-of-pack symbols were voluntary, non-standardized measures used by brands (such as Campbell Soup) to boost transparency or competitive positioning.
To help consumers easily identify healthier choices, the FDA is proposing a standardized front-of-pack (FOP) nutrition label. Unlike the voluntary Nutri-Score or UK Traffic Light systems, this standardized U.S. framework aims to provide immediate clarity on key nutrients, ensuring consistency across all packaged goods.
Voluntary Facts Up Front Labeling
The need for FDA FOP labeling can be attributed to the rising popularity of voluntary FOP labeling like Facts Up Front, a labeling initiative led by the Consumer Brands Association (formerly the GMA) and FMI – The Food Industry Association. These two associations share a common goal – supporting consistent food labeling practices while ensuring consumers have clear nutrition information.
This existing label displays a snapshot of the Nutrition Facts panel on the front of the pack, typically highlighting calories, saturated fat, sodium, and added sugars. While some brands also include “nutrients to encourage”, like fiber or potassium, the program remains entirely optional.
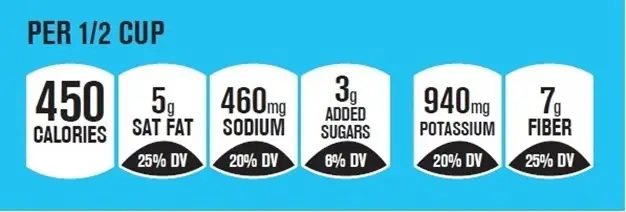
Example of a Voluntary Facts Up Front Label
Below you’ll find examples of Facts up Front Labels highlighting information about calories, saturated fat, sodium, and added sugars, which are dietary components the Dietary Guidelines for Americans recommend limiting.
Because there are no mandatory rules for colors or layout, participation has been inconsistent across the market. And while Facts Up Front helped consumers find basic data quickly, its lack of standardization is exactly what the FDA’s proposed FOP nutrition label aims to solve.


Why the FDA Is Moving Toward a Mandatory FOP Label
In January 2025, the FDA issued a proposed rule to set a standardized order for a proposed FOP nutrition label on most packaged foods – highlighting saturated fat, sodium, and added sugars.
The shift toward a mandatory proposed FOP nutrition label is driven by a simple reality: consumers are making purchasing decisions in seconds. 70% of Americans say that front-facing labels make it significantly easier to select healthy food and beverage products, with 85% of consumers expressing high satisfaction with the “Facts Up Front” style of labeling. With 60% of Americans living with a preventable chronic disease, such as cardiovascular disease, diabetes, or obesity. To combat this rising tide of illness and premature death, the Food and Drug Administration (FDA) is shifting its focus toward simplicity – empowering consumers to identify healthier food choices in the seconds it takes to scan a shelf.
The FOP proposal is also a key deliverable of the White House National Strategy on Hunger, Nutrition, and Health. It’s designed to foster healthy dietary patterns across the country. Research from the Food and Drug Administration shows that while the side-panel Nutrition Facts label is comprehensive, it is rarely consulted during “grab-and-go” shopping.
While voluntary industry efforts like Facts Up Front improved visibility, their inconsistent adoption has created a fragmented marketplace where consumers are not able to compare products easily.
By standardizing a “Nutrition Info” box, the FDA aims to help shoppers easily identify what they’ve called “nutrients of concern,” specifically saturated fat, sodium, and added sugars. This mandate is a cornerstone of the National Strategy on Hunger, Nutrition, and Health, designed to reduce diet-related chronic diseases. By moving to an interpretive model (using “High,” “Medium,” and “Low” markers), the U.S. is finally aligning with successful global systems to provide nutritional transparency at a glance.
The Proposed FOP Timeline
While the front of pack nutrition labeling rule is not yet finalized, the industry expects a transition period of 3 to 4 years once the final version is published. This gives manufacturers time to reformulate and redesign.
It’s also worth noting that while the FDA oversees most packaged goods, the USDA maintains separate voluntary and mandatory FOP guidelines for meat and poultry. Staying ahead of these dietary guidelines now will ensure your brand is ready when the “voluntary” era officially ends.
FDA’s Proposed Nutrition Info Box
From a visual perspective, the Nutrition Info Box is designed as a compact informational box to help consumers quickly and easily identify a product’s health profile at the point of purchase.
Designed to sit prominently in the top third of the Principal Display Panel (PDP), this new box serves to complement the detailed Nutrition Facts panel rather than replace it, providing immediate transparency and at-a-glance information.
Core Nutrient Information
As mentioned earlier, the 2025 FOP proposal focuses exclusively on three primary nutrients: saturated fat, sodium, and added sugars. These were chosen because they are closely linked to chronic health conditions like heart disease and type 2 diabetes.
Under this system, each is categorized as Low, Med, or High based on its % Daily Value (%DV) per serving.
Manufacturers may voluntarily highlight positive nutrients (like fiber or protein) elsewhere on the package, provided all values remain consistent with the Nutrition Facts panel. While calories are not a mandatory requirement in this new format, the FDA allows brands to include them voluntarily.
Example of the Proposed Nutrition Info Box
To provide a clear visual of the upcoming regulatory shift, we’ve included a sample of the proposed FOP nutrition label. This demonstrates the standardized Nutrition Info Box design that is central to the FDA’s new transparency requirements.
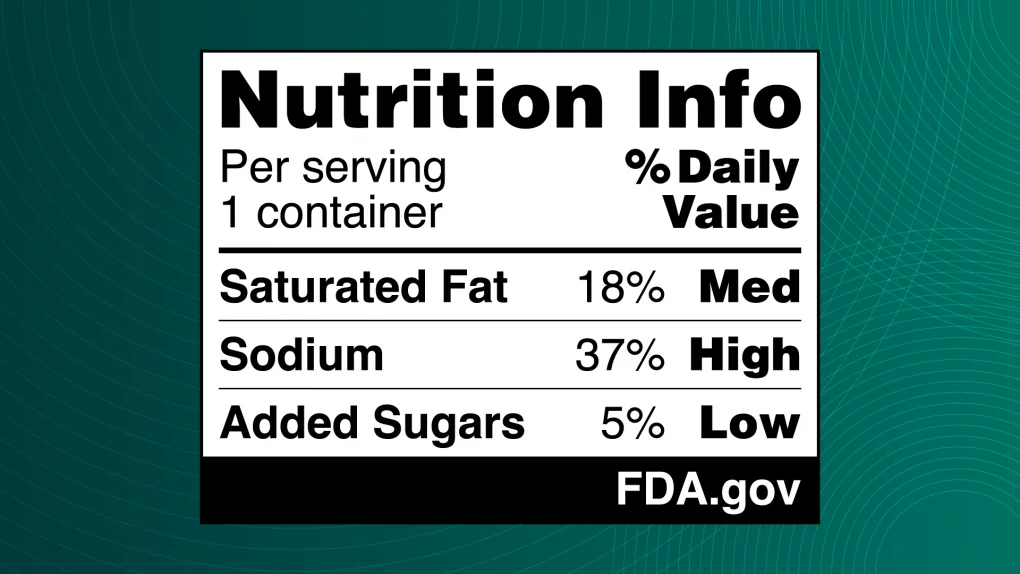
Interpretive vs. Non-Interpretive Formats
Nutrition labels generally fall into two categories: non-interpretive and interpretive.
Non-interpretive formats provide raw data, such as numeric calorie counts or gram values, leaving the consumer to determine if those levels are healthy.
In contrast, interpretive formats use visual cues or text like “Low Sodium”, or the FDA’s proposed “Low/Med/High” ratings to provide immediate context. While the FDA has not adopted international “Traffic Light” colors or warning symbols, this new standardized scheme serves as an interpretive tool to help consumers evaluate product healthfulness without needing deep nutritional knowledge.
Claims and Statements Allowed on the FOP Panel
The front-of-pack label is also the primary home for Nutrient Content Claims, regulated under 21 CFR 101. Claims like “Good Source of Fiber” or “Reduced Fat” must meet specific FDA definitions and align exactly with the mandatory Nutrition Facts panel.
While these voluntary claims are permitted alongside the new Nutrition Info Box, they do not imply an official FDA endorsement of the product’s overall healthfulness.
See How FoodLabelMaker Can Help You
Format and Presentation of Front-of-Pack Labeling
The visual structure of a food package plays a decisive role in how shoppers interpret health data. Under current voluntary systems, package nutrition labeling on the front of the pack is quite flexible, given the claims are truthful and non-misleading. However, the 2025 proposal marks a shift toward greater standardization of format and placement.
If finalized, the Nutrition Info Box will have legally defined formatting and placement rules, moving away from the varied designs seen in today’s food labeling to ensure universal legibility and consistency across all retail categories.
Label Design and Layout
Every Principal Display Panel (PDP) must legally feature two primary elements:
- The Statement of Identity (the product’s common name)
- The Net Quantity of Contents
These mandatory features must remain the most prominent items on the food package; they cannot be overshadowed or displaced by any front-of-pack graphics.
Currently, voluntary FOP elements have flexible placement on the PDP, provided they are grouped for clarity and don’t interfere with required information. However, under the FDA’s 2025 proposed rule, the standardized Nutrition Info Box would be restricted to the top third of the PDP to ensure it’s easy for consumers to find.
Beyond placement, the box needs to be consistent. Every nutrient value and serving size displayed on the front must match the Nutrition Facts panel exactly.
Furthermore, FDA guidance dictates that voluntary systems must be truthful and accurate, never obscuring the information panel or using designs that falsely imply an official government endorsement.
By maintaining this hierarchy, manufacturers ensure that secondary nutritional cues never compromise the legal integrity of the primary food labeling. For example, secondary nutritional cues include nutrient content claims like “low fat” or dietary guidelines statements such as “Eating whole grains as part of a healthy diet may help reduce the risk of heart disease.”
Color and Contrast Requirements
The FDA requires all food labeling to meet strict contrast and legibility standards, ensuring that essential data is easily readable for every consumer.
While some global models, like the U.K. and EU, use “traffic-light” colors, it is important to note that the FDA does not endorse or regulate these systems; any color used must not imply official government approval.
On the Principal Display Panel (PDP), all voluntary FOP features must maintain high visibility and a distinguishable contrast. Crucially, these colors must not obscure or compete with mandatory elements, such as the statement of identity and net quantity, which must always remain clear.
Font Size and Legibility Rules
When it comes to font size, the FDA has very specific font regulations for how large your text needs to be, and while these rules technically apply to mandatory elements like the Statement of Identity and Net Quantity, they are also the benchmark for any voluntary front-of-pack info added.
| Label Element | Minimum Font Size Requirements | Key Formatting Rule |
| Statement of Identity | Must be at least 1/2 the size of the largest print on the label. | Must be in Bold type and parallel to the base. |
| Net Quantity (Contents) | Scales by package size (see below). PDP Surface Area and Minimum Type Size (lowercase ‘o’)Small: ≤ 5 in² = 1/16 inchSmall packages (≤ 5 in² PDP): minimum type size of 1/16 inchStandard packages (> 5 to ≤ 25 in² PDP): minimum type size of 1/8 inchMedium packages (> 25 to ≤ 100 in² PDP): minimum type size of 3/16 inchLarge packages (> 100 to ≤ 400 in² PDP): minimum type size of 1/4 inchOversized packages (> 400 in² PDP): minimum type size of 1/2 inch | Must be in the lower 30% of the PDP. |
| Nutrition Facts Title | Largest text on the panel (except for the calorie number). | Must be in Bold and span the full panel width. |
| Calories (Heading) | 16 point (10 point for small packages). | Must be in Bold. |
| Calories (Number) | 22 point (14 point for small packages). | Usually the largest number on the package. |
| Nutrients (e.g., Fat, Sodium) | 8 point (minimum). | Nutrient names are usually in Bold. |
| Manufacturer & Ingredients | 1/16 inch (based on lowercase “o”). | Must be easy to read and high-contrast. |
Best Practices for Front-of-Pack Nutrition Labeling
If you’re adding a voluntary Nutrition Info Box or other callouts, here’s how to stay compliant with the FDA’s regulations:
- Don’t Crowd the PDP: Keep enough “clear space” around your text. If it looks cluttered, it’s likely non-compliant.
- Avoid “Official” Mimicking: Don’t style your voluntary text to look like a government warning or official endorsement. Stick to clean, legible fonts like Helvetica or Arial.
- The Mirror Rule: Your front-of-pack numbers must match the Nutrition Facts panel. If your information panel says “10% DV,” your front panel cannot use a larger, bolder font that makes “10%” look more significant than it actually is.
Calculating and Displaying Portion Sizes on Food Packaging
Determining the right numbers for your FOP nutrition labeling starts with a clear understanding of the FDA’s serving size regulations. It is essential to distinguish between a “portion” (the amount a person actually chooses to eat) and a “serving size,” which is the standardized amount defined by the FDA’s Reference Amounts Customarily Consumed (RACC). RACC values allow consumers to compare similar packaged foods and encourage healthy food decisions.
Consider these common scenarios:
- Granola Bars: Usually a single-serve item where one bar equals one serving.
- Chips: Typically a multi-serve product; the FOP label must reflect the RACC (often 28g or 1 oz) rather than the entire bag.
- Beverages: A 20 oz soda is often labeled as one serving because it’s typically consumed in one sitting, whereas larger bottles may require dual-column labeling.
If the FDA’s new rule is finalized, these same strict serving size standards will remain the mandatory foundation for the Nutrition Info Box, ensuring that front-of-pack snapshots never contradict the detailed panel on the back.
Front-of-Pack Labeling for Different Product Types
While the goal of front-of-package nutrition labeling is consistency across food packages, it’s important to remember that different types of packaged foods need their own set of regulations. Because the FDA is still in the rulemaking phase, the proposed framework offers early insight into how different product categories may be treated.
By understanding these nuances now, brands can begin auditing their packaging to ensure a smoother transition once a final rule is published.
Packaged Foods
For standard packaged foods, the focus remains on distinguishing between single-serve and multi-serve items.
- Dual Columns: Under the current proposal, the “Nutrition Info” box would reflect “per serving” values. For brands using dual-column labeling (like a pint of ice cream showing “per serving” and “per container”), you would likely need to align your front-of-pack data with the single-serving column.
- Small Packages: If your product requires a very small package (under 12 sq. inches), you may be exempt from bearing the mandatory box. However, if a food manufacturer still opts to add a voluntary claim, they are no longer exempt, and the claim would need to be 100% accurate and compliant.
Beverages
Beverages are predicted to be a high-priority category for the FDA due to added sugars and the fact that the beverage industry has long been a leader in voluntary front-of-pack transparency.
For example, the American Beverage’s Clear on Calories initiative ensures that 100% of their member companies’ products (including Coca-Cola) provide calorie information at the consumer’s fingertips.
Some expected requirements from the FDA on front-of-pack regulations for beverages include:
- Calorie Display Requirements:
- For single-serve containers (like a 20 oz soda), FOP nutrition labels often show the total calories per bottle.
- For larger, multi-serve containers, you can show calories per serving.
- The key is that whatever you put on the front must match the Nutrition Facts panel exactly.
- Sugar-Sweetened Beverage Considerations: Because added sugars are a major focus for a healthy diet, the FDA’s proposal encourages clear callouts. Many brands are already using voluntary FOP nutrition labeling to highlight “Low Sugar” or “0g Added Sugars” to help guide consumers
- Regulated Claims: If you want to use claims like “low calorie,” “reduced sugar,” or “contains electrolytes,” you must meet the specific criteria under 21 CFR 101. These can appear on the front of the pack, but they must be substantiated by information panel data.
Dietary Supplements
Current drafts of the proposed FOP nutrition label specifically exclude dietary supplements. Because supplements follow “Supplement Facts” regulations rather than “Nutrition Facts,” they are not expected to be required to carry the new Info Box.
A key difference is in the types of claims allowed: while conventional food labels focus on nutritive value, dietary supplements often feature “structure/function” claims (such as “supports bone health” or “boosts immunity”).
However, if you choose to add voluntary callouts to a supplement, they must stay distinct so they don’t confuse consumers into thinking the product is a conventional food.
Multi-Component or Variety Packs
To help consumers maintain a healthy diet, the FDA requires that nutrition labeling for these packaged foods remain consistent across all layers of packaging.
- Mini packs and multipacks: Like a six-pack of yogurt – the front-of-pack (FOP) information can be shown either “per unit” (one cup) or “per package” (the entire six-pack). However, to avoid confusion, the label must clearly state which approach is being used. If you opt for per-package totals, you should include the total number of servings to maintain consistency with the Nutrition Facts panel.
- Outer vs. inner packaging requirements: While the outer packaging acts as the primary source of information, inner packages may still require their own Nutrition Facts panels if they can be sold individually. Crucially, any FOP display on the outer wrap must not contradict or obscure the mandatory labeling on the inner units, ensuring transparency from the shelf to the table.
Benefits of Implementing Front-of-Package Nutrition Labels
Adopting the FDA’s proposed front-of-pack nutrition label presents an opportunity for brands to demonstrate transparency and preparedness as dietary guidance evolves toward at-a-glance nutrition information.
Enhanced Consumer Understanding and Transparency
Shoppers spend an average of only 35 seconds looking at a food product before deciding to place it in their basket. This small window doesn’t give enough time for consumers to study the fine print on packaged foods. By providing front-of-package nutrition information, consumers can quickly interpret key nutrients at a glance.
Support For Health-Focused Brand Positioning
Aligning what’s communicated on a food package with evolving dietary guidelines sends a clear message to consumers that your brand is up-to-date and transparent. It also signals that as a food manufacturer, you’re supporting healthier diet decision-making. More health-conscious shoppers are more likely to seek out your brand without hesitation.
Reduced Customer Inquiries
Confusion leads to questions, and questions lead to customer service tickets. When nutritional data is buried or difficult to read, brands often see a high volume of inquiries regarding calorie counts, allergens, or specific dietary attributes.
An easy-to-read nutrition info box provides the answers upfront. By addressing information gaps at the point of purchase, you reduce the burden on your support team and provide a smoother experience for the end user.
Competitive Advantage for Early Adopters
In an increasingly competitive market, such as the food industry, standing out is the key to survival and success. 79% of Americans check front-of-pack labels before they buy a food item, so brands that prepare early for front-of-pack labeling may be better positioned to adapt quickly once requirements are finalised Food manufacturers that gain this competitive advantage are more likely to capture the attention of health-focused consumers and gain their long-term trust.
Alignment With Future FDA Requirements
The FDA’s 2025 proposal suggests a compliance window of three to four years once a final rule is published. Choosing to implement a proposed nutrition info box style now means your team is already “compliance-ready” when the law officially changes.
This early alignment prevents the frantic, last-minute redesigns that often lead to supply chain disruptions, allowing your brand to transition smoothly while others are still catching up.
At Food Label Maker, we closely monitor every FDA update to ensure our community is the first to know when new mandatory regulations are published. In the meantime, you can ensure your products are 100% compliant with today’s regulations using our intuitive nutrition labeling platform. Create a free label or view our pricing plans.
Common FOP Mistakes and How to Avoid Them
Transitioning to front-of-pack labeling leaves little room for error as brands can face FDA issues or warning letters if their FOP labels aren’t aligned with FDA regulations. Although FOP labeling is still a proposal and hasn’t been finalized, we can expect similar labeling mistakes to appear in information panels.
More often than not, these mistakes stem from inconsistent labeling practices and an incomplete understanding of FDA labeling laws.
By identifying these common pitfalls early on, you can ensure that your FOP labels are clear, compliant, and serve their intended purpose of helping consumers make healthier food choices.
Incorrect Serving Size Calculations
A major source of non-compliance is using “marketing-friendly” portions instead of legally regulated serving sizes. Human error in manual math manual calculation errors or a misunderstanding of FDA Reference Amounts Customarily Consumed (RACC) can lead to FOP values that are factually incorrect.
- Mistake: Using “marketing-friendly” portions instead of FDA-defined serving sizes. Manual calculation errors or misunderstandings of Reference Amounts Customarily Consumed (RACC) can lead to inaccurate front-of-pack values.
- Solution: Always anchor your data to the official FDA RACC for your specific food category. Ensure front-of-pack values align with the Nutrition Facts panel.
Misuse of Nutrient Claims
It is surprisingly easy to misapply terms like “Low Sodium,” “Reduced Sugar,” or “Good Source” on the front of a pack.
- Mistake: If your product doesn’t meet the strict per-serving thresholds defined in 21 CFR 101, the claim is illegal.
- Solution: Verify your product’s eligibility for every specific claim before printing. For example, a “High Protein” claim must contain 20% or more of the Daily Value (DV) of protein per Reference Amount Customarily Consumed (RACC). Always cross-check your lab results against current FDA thresholds to ensure all claims are properly substantiated.
Using Traffic-Light Colors Incorrectly
While “Green-Amber-Red” color-coding systems are common in other markets, like the FSA, the FDA’s 2025 proposal favors a neutral, non-interpretive design.
- Mistake: Using colors that imply a “stop/go” rating or an FDA endorsement can be flagged as misleading.
- Solution: Stick to high-contrast, neutral designs (typically black and white). Avoid interpretive color-coding that could overstate a nutrient’s meaning or confuse shoppers who have lower nutrition knowledge.
Mismatched Values Between FOP and Nutrition Facts
One of the fastest ways to lose consumer trust is by not having all nutrition data consistent across food packages. These discrepancies often happen due to inconsistent rounding or outdated data during a package redesign.
- Mistake: Having a calorie or nutrient count on the front that doesn’t match the information panel.
- Solution: Implement a “single source of truth” for your data. Your FOP snapshot must match the rounding increments and values on your Nutrition Facts panel exactly.
Poor Contrast, Layout, or Legibility
If a shopper can’t read your label in that critical 35-second window, it has failed.
- Mistake: Using low-contrast text (like white text on a light background) or fonts that are too small to be legible on a crowded Principal Display Panel (PDP).
- Solution: Prioritize high contrast and follow FDA font-size best practices. A clean, uncluttered layout ensures that consumers can comprehend FOP nutrition information at a glance without straining.
Placement That Interferes With Mandatory Labeling
Voluntary FOP icons should never compete with the information the law requires.
- Mistake: Placing a calorie icon so large or so close to the Statement of Identity (the product name) that it obscures the mandatory PDP hierarchy.
- Solution: Position your FOP content so that the product name and Net Quantity of Contents remain the most prominent features. Avoid overlapping required information and follow standard PDP layout rules to keep your label legally sound.
While the FDA finalizes the new ‘Nutrition Info’ box requirements, you can ensure your Nutrition Facts labels are accurate and compliant. Use Food Label Maker to generate precise, compliant Information Panels that will make your eventual transition to FOP labeling seamless.
Final thoughts: FDA Front of Packages Labeling
The shift toward mandatory front-of-pack labeling represents one of the most significant changes in the FDA towards food transparency in decades.
By moving critical data from the side Information Panel to the Principal Display Panel, the FDA aims to make nutrition information more accessible at the point of purchase. This evolution is essential for helping consumers align their daily choices with modern dietary guidelines and maintain a healthy diet in a fast-paced world.
For brands, preparing for the proposed FOP nutrition label framework supports consumer trust and long-term compliance. While the Nutrition Info Box is still under review, prioritising clear, accurate nutrition labeling now helps position packaged foods as transparent and credible.
Whether you’re preparing for future FDA FOP requirements or need to generate a 100% accurate Nutrition Facts label today, our platform is built to help you navigate the complexities of FDA regulations with ease. Create a free label or view our pricing plans.


